In the current electronic and optoelectronic industries, as electronic devices and products evolve towards higher integration and computing power, dissipated power has doubled. Heat dissipation has gradually become a key factor limiting the sustainable development of the electronic industry. Finding heat management materials with excellent thermal conductivity is crucial for designing next-generation integrated circuits and three-dimensional electronic products. The thermal conductivity of traditional ceramic materials, such as boron nitride and aluminum nitride, and metal materials, like copper and aluminum, is only a few hundred W/(m·K) at most. In contrast, carbon materials, such as diamond, graphite, graphene, carbon nanotubes, and carbon fiber, offer much higher thermal conductivity. For example, the thermal conductivity of graphite in the direction parallel to the crystal layers can theoretically reach 4180 W/m·K. This is almost 10 times that of traditional metals like copper, silver, and aluminum.

Additionally, carbon materials also have excellent properties like low density, low thermal expansion coefficient, and good high-temperature mechanical properties.
In short, carbon materials have a unique heat conduction/dissipation capability, which makes them the most promising heat dissipation materials in recent years.
Thermal conductivity of different carbon materials
Graphene
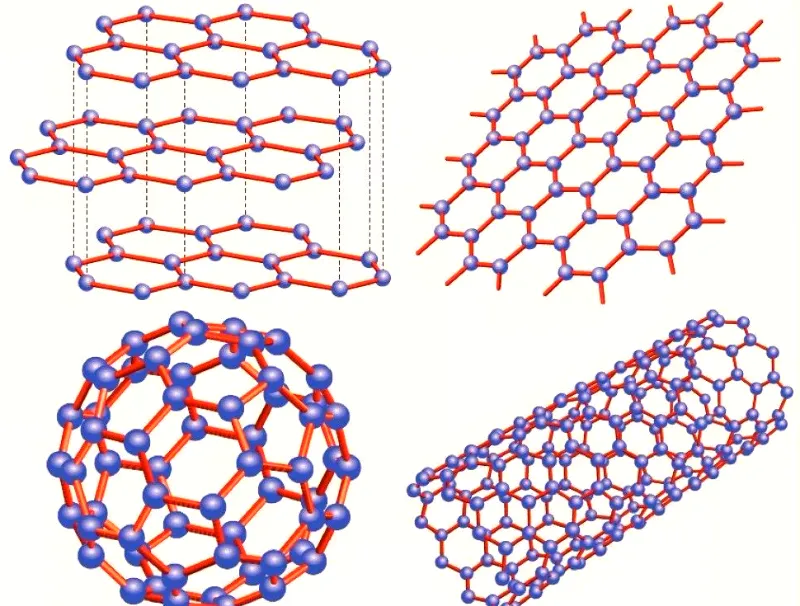
Graphene is a material composed of a single layer of carbon atoms exfoliated from graphite. Its structure forms a hexagonal lattice, creating a stable, honeycomb-shaped two-dimensional plane. The carbon atoms within graphene are connected with great flexibility. When external force is applied to graphene, the carbon atom plane bends and deforms, allowing the carbon atoms to maintain structural stability without rearranging. This stable lattice structure endows graphene with exceptional thermal conductivity.
Studies have shown that the thermal conductivity of single-layer graphene at room temperature can reach 3000 to 5300 W/(m·K).
Carbon Nanotubes
Since the discovery of carbon nanotubes in 1991, they have been a focal point. Many researchers have studied their thermal conductivity. Carbon nanotubes are formed by curling single or multiple layers of graphite sheets. They can be categorized into single-walled, double-walled, and multi-walled types.
The unique structure of carbon nanotubes gives them exceptionally high thermal conductivity. Studies have shown that the thermal conductivity of single-walled carbon nanotubes at room temperature is 3980 W/(m·K). Double-walled carbon nanotubes have a thermal conductivity of 3580 W/(m·K). Multi-walled carbon nanotubes have a thermal conductivity of 2860 W/(m·K).
diamond

Diamond has a crystal structure where carbon atoms are closely arranged in a tetrahedral pattern. All electrons are involved in bonding, which results in an exceptional thermal conductivity of 2000–2100 W/(m·K) at room temperature. This makes diamond one of the best materials for thermal conductivity in nature. This property makes it irreplaceable in high-end heat dissipation applications.
Carbon Fiber
Carbon fiber is formed by high-temperature carbonization, resulting in a disordered graphite structure. When its axial graphite lattice is highly oriented, it can achieve ultra-high thermal conductivity. For example, the thermal conductivity of pitch-based carbon fiber reaches 1100 W/(m·K), while that of vapor-grown carbon fiber can reach 1950 W/(m·K).
Graphite
Graphite belongs to the hexagonal crystal system, composed of six prismatic faces and two densely packed basal planes. The carbon atoms in the first layer form a hexagonal lattice, with the second layer staggered by half the diagonal of the hexagon, and they overlap in parallel. The third layer repeats the position of the first, creating an A-B-A-B… sequence. The thermal conductivity of natural graphite along the (002) crystal plane is 2200 W/(m·K). The in-plane thermal conductivity of highly oriented pyrolytic graphite can also reach 2000 W/(m·K).
conclusion
Carbon materials have unique crystal structures and physicochemical properties. These materials show irreplaceable advantages in thermal conductivity and heat dissipation. With advances in preparation technologies and expanding application scenarios, carbon-based materials, such as graphene and diamond, are expected to improve thermal management solutions. This will help industries like electronics and aerospace reach a higher level.
Epic powder
Epic Powder, 20+ years of work experience in the ultrafine powder industry. Actively promote the future development of ultra-fine powder, focusing on crushing, grinding, classifying and modification process of ultra-fine powder. Contact us for a free consultation and customized solutions! Our expert team is dedicated to providing high-quality products and services to maximize the value of your powder processing. Epic Powder—Your Trusted Powder Processing Expert !

