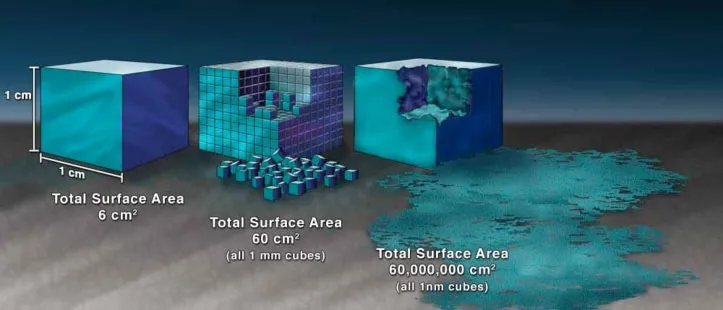
Generally, powders with a particle size smaller than 1 μm are defined as ultrafine powder. Ultrafine powder exhibit surface and volume effects distinct from the original material or coarser powders. These powders show different properties compared to larger particle powders.

Surface Effect
The main difference between ultrafine powder and macroscopic objects is the increased surface atoms. Ultrafine powders have a large specific surface area. The surface effect becomes significant and cannot be ignored. Surface atoms differ from internal atoms in their physical properties. Internal atoms are surrounded symmetrically by other atoms. Surface atoms are asymmetrically positioned and are attracted by internal atoms. This results in higher energy for surface atoms compared to internal atoms.
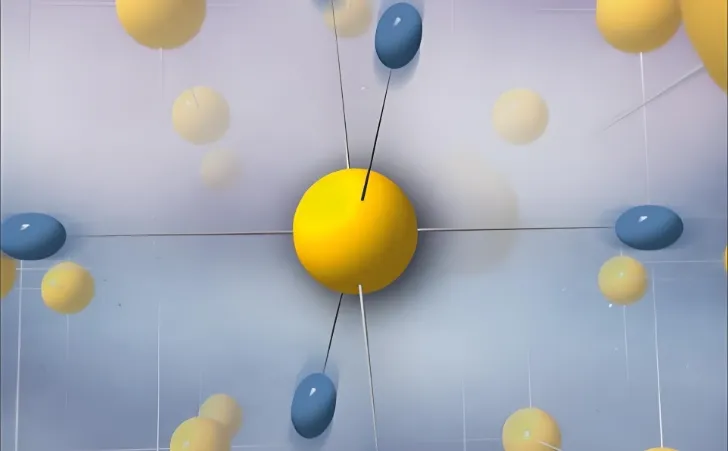
Quantum effect
This refers to the phenomenon when particle size decreases to a certain point. Electrons near the metal Fermi level transition from quasi-continuous to discrete. According to solid-state band theory, conducting electrons move in a periodic potential field. These electrons no longer belong to individual atoms but to the entire crystal. This “public” behavior results in quasi-continuous energy bands in the crystal. The energy difference between adjacent levels is much smaller than thermal energy.
Optical properties
The color of metal particles is often different from that of bulk materials. When the size of the metal particles is less than a certain value, they usually appear black due to the total absorption of light waves. In addition to the absorption of light waves, ultrafine particles also have a scattering effect.
For ultrafine dispersed particles smaller than a few tenths of the wavelength of light, the intensity of scattered light is inversely proportional to the fourth power of the wavelength. Therefore, the scattering of sunlight by the dust in the atmosphere makes the clear sky blue.
The ultrafine clay solution highly dispersed in water, when viewed from the side against a dark background, appears blue-white, as if it is a bit turbid. In fact, this is the result of the ultrafine clay particles in the solution scattering part of the incident light.
Electrical properties
Metallic materials have conductivity, but the conductivity of nano-metal particles is significantly reduced. When the electric field energy is lower than the interval of the splitting energy level, the conductivity of the metal will be transformed into electrical insulation.
Magnetic properties
The magnetic properties of ultrafine powder, especially ferromagnetic particles, have long been a research focus.
For bulk magnetic materials, multiple magnetic domains usually form in the neutral state. Each domain’s magnetic moment aligns with the direction of its lowest energy. There is a transition layer between domains where magnetization direction changes continuously, called a magnetic wall.
The chaotic arrangement of magnetic domains follows the principle of minimum energy for the entire ferromagnet. This causes macroscopic magnetization to be zero in the magnetic neutral state. The magnetic domain orientation typically depends on the type of magnetic anisotropy.
Magnetic ultrafine powders have wide applications in recording media. Examples include γ-Fe2O3, FeCo metal, CrO2, TixCOxO19, BaFe12-2x, Fe4N, and Co-γ-Fe2O3. As magnetic fluids, nano ferrites like Fe3O4, and nano particles of iron, nickel, cobalt, and alloys are used. In magnetic fluid applications, microparticles must be coated with organic long-chain molecules.
Due to their small size and large surface area, the coating greatly affects their magnetic properties.
Thermal properties
The change in particle size alters the specific surface area. This changes the chemical potential of the particles. As a result, the thermodynamic properties are affected.
Particle size has a significant impact on thermodynamic properties. As particle size decreases, surface energy increases significantly. This allows ultrafine powders to melt or sinter at lower temperatures. The temperature required is lower than the melting point of bulk material.
Catalytic properties
For heterogeneous catalytic reactions, reducing particle size is necessary to increase the specific surface area and improve catalytic efficiency.
However, it is not the only factor. Some catalysts show peak catalytic efficiency at specific particle sizes. Therefore, studying the impact of particle size and surface condition on catalytic activity is essential.
Mechanical properties
For traditional metal materials, hardness increases with grain refinement. For coarse-grained metals, their mechanical properties improve as grain size decreases.
For some pure metal nanoparticles, such as palladium, copper, silver, nickel, and selenium, microhardness increases significantly at room temperature compared to their coarse-grained counterparts.
However, for intermetallic compounds, hardness decreases as grain size decreases below a certain critical size. In nanomaterial research, the mechanical properties of nanoceramics are of great interest.
Nanoceramics have advantages such as high temperature resistance, high hardness, and chemical stability. Their drawbacks include poor machinability, brittleness, and lack of ductility. Nanoceramics are expected to overcome these shortcomings.
Magnetoresistive properties
The so-called magnetoresistance effect refers to the change in resistivity caused by a magnetic field. To achieve a large magnetoresistance effect, the particle size or thickness of magnetic and non-magnetic layers must be smaller than the average free path of electrons. This minimizes scattering during electron transport, apart from spin-related scattering, and allows spin orientation to remain unchanged. Since the average free path of electrons is typically a few nanometers to 100 nm, giant magnetoresistance can only be observed in nanoscale systems.
Solution properties
Motion of ultrafine particles in solution:
In solutions or suspensions with ultrafine powder particles as solutes, these particles diffuse from high concentration to low concentration areas. At the same time, Brownian motion also occurs.
Adsorption of ultrafine particles in solution:
Adsorption is one of the interfacial phenomena that occurs between interacting phases. It refers to the phenomenon where adsorbates are held in a thin contact layer on the interface or surface of the adsorbent. With a large specific surface area, high surface energy, and high adsorption capacity, ultrafine particles exhibit significant adsorption.
Rheology:
Rheology is the science of studying the flow and deformation of materials. As discussed earlier, as particle size decreases, particles gradually exhibit different properties or behaviors compared to the original solid.
Particles smaller than 1 μm in liquid dispersion, known as colloidal or particle dispersion systems, are both theoretically and practically significant research subjects in rheology.
Conclusion
In conclusion, the unique characteristics of ultrafine powder, including its high surface area, increased reactivity, and enhanced mechanical properties, make it a valuable material across various industries. These properties enable ultrafine powders to meet the demands of advanced applications in fields like catalysis, material science, and nanotechnology. As research and technology continue to evolve, the potential of ultrafine powders will expand, offering new opportunities for innovation and performance enhancement.
epic powder
Epic Powder, 20+ years of work experience in the ultrafine powder industry. Actively promote the future development of ultra-fine powder, focusing on crushing,grinding,classifying and modification process of ultra-fine powder. Contact us for a free consultation and customized solutions! Our expert team is dedicated to providing high-quality products and services to maximize the value of your powder processing. Epic Powder—Your Trusted Powder Processing Expert !

