Lithium batteries are a vital energy source for modern technology, powering devices such as smartphones, laptops, electric vehicles, and energy storage stations. The performance of these batteries is closely linked to the selection and properties of their materials. The key Lithium Battery Materials include the anode, cathode, separator, and electrolyte. Each of these components plays a crucial role, working in synergy to enable the efficient charging and discharging processes that make lithium batteries essential for various applications.
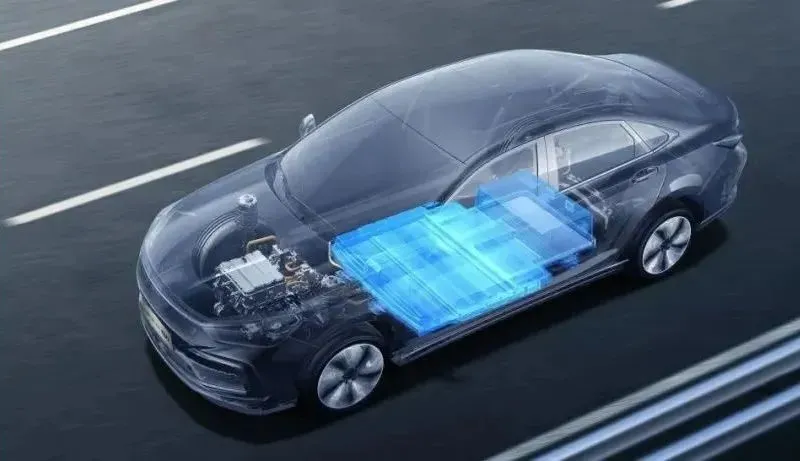
Cathode Materials
Cathode materials play a key role in lithium batteries, accounting for about 40% of the battery cost. They directly determine the energy density of the battery. Different types of cathode materials have their own characteristics and play important roles in various fields.
Lithium Cobalt Oxide (LCO)
Widely used in early mobile phones, laptops, and other small electronic devices, LCO offers high working voltage and energy density, providing stable power support for devices. However, cobalt is scarce and expensive. Additionally, LCO has poor safety performance under high temperature or overcharge conditions, limiting its large-scale application.
Lithium Manganese Oxide (LMO)
LMO has a lower cost and better safety but offers lower energy density and limited cycle life. It is used in applications that are cost-sensitive and don’t require high energy density, such as electric bicycles, some low-end electric vehicles, and energy storage systems.
Lithium Iron Phosphate (LFP)
Known for its high safety, long cycle life, and low cost, LFP has good thermal stability. It is less prone to thermal runaway even in high-temperature environments, greatly reducing the risk of battery fires or explosions. LFP is widely used in power batteries for new energy vehicles, especially commercial vehicles and energy storage stations with high safety requirements. With advancements in technology, its energy density continues to improve, and its application range is expanding.
Nickel Cobalt Manganese (NCM) and Nickel Cobalt Aluminum (NCA)
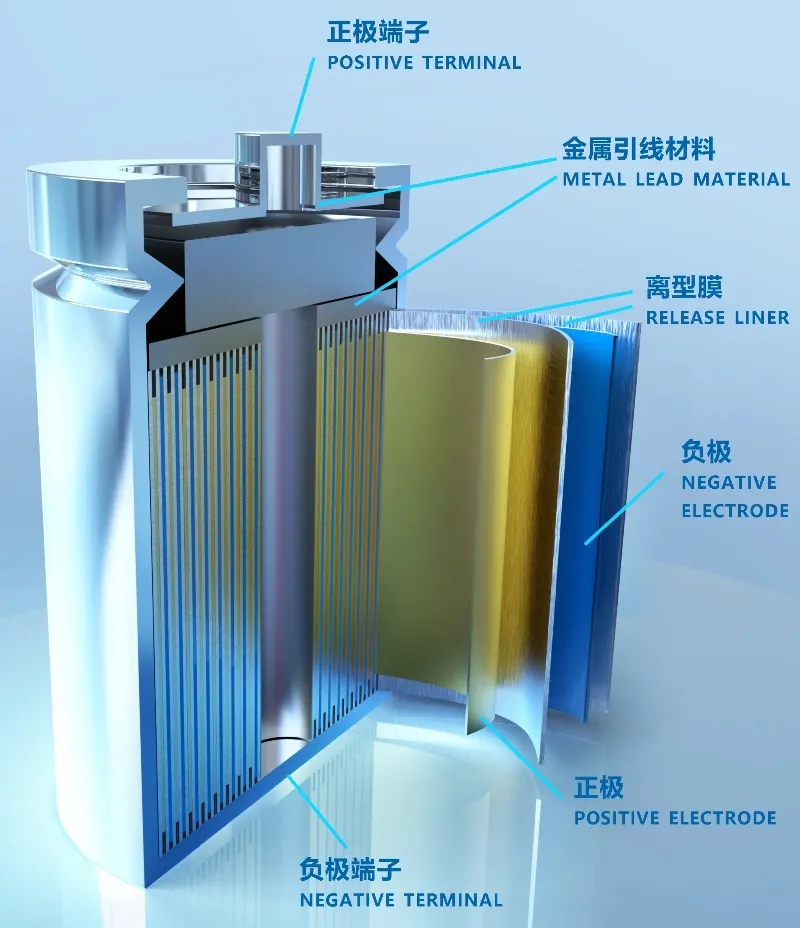
By adjusting the ratio of nickel, cobalt, and manganese (or aluminum), these materials balance energy density, cycle life, and safety. Nickel increases battery capacity, manganese ensures safety, and cobalt enhances cycle performance. High-nickel ternary materials (such as NCM811 and NCA) offer high energy density and provide electric vehicles with longer ranges, making them popular in the passenger car market. However, their safety and stability are slightly lower compared to LFP batteries.
Anode Materials
Currently, lithium battery anode materials mainly consist of natural graphite and synthetic graphite. Both materials have high crystallinity, stable layered structures, low lithium insertion potential, and flat plateaus. These advantages enable them to provide high specific capacity and good cycle performance, meeting the daily usage requirements of lithium batteries.
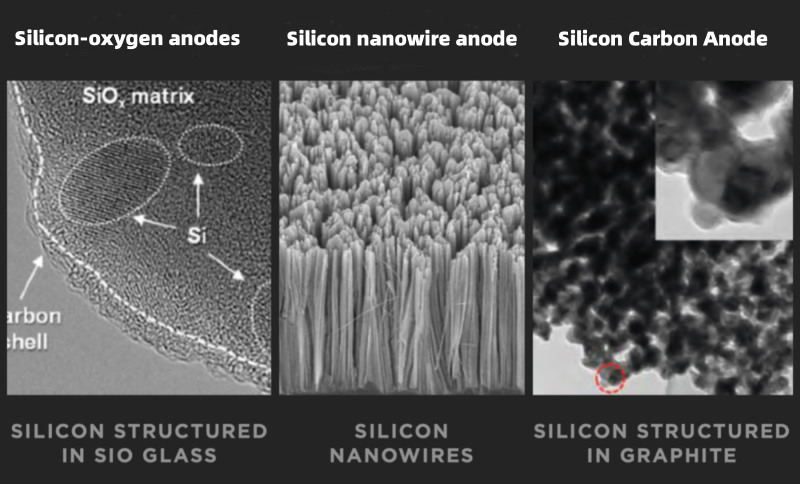
At the same time, researchers are actively exploring new anode materials. Nitride, tin-based oxides, tin alloys, and other materials, while having higher theoretical capacities, suffer from issues like large volume expansion and poor conductivity. These problems result in poor cycling stability, and they have not yet been commercialized.
Nanomaterials for anodes, such as carbon nanotubes and nano-alloys, show better electrochemical performance due to their special size and surface effects. They offer higher specific capacity, faster charge-discharge rates, and better cycling stability. Many companies have started adding these materials to traditional anode materials to improve the overall performance of lithium batteries.
Separator
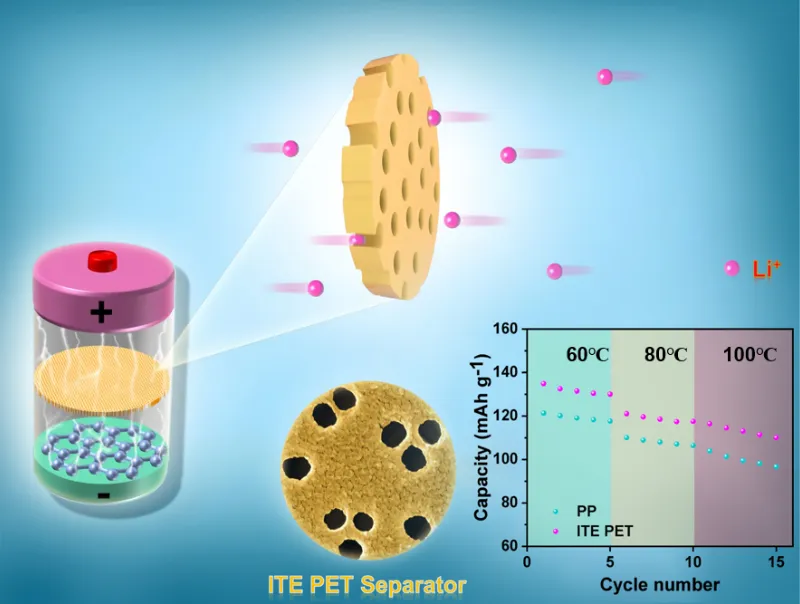
Commercial separators are primarily made from polyolefins, such as polyethylene (PE) and polypropylene (PP). They play a crucial role in the lithium battery structure. The separator forms a physical barrier between the anode and cathode, preventing direct contact that could cause a short circuit. At the same time, it allows lithium ions to pass through, ensuring ion conduction within the battery.
The performance of the separator significantly impacts the battery’s safety, cycle life, and charge-discharge performance. Parameters such as pore size, porosity, and thickness need to be precisely controlled. The appropriate pore size and porosity allow smooth lithium ion passage while blocking electron conduction to prevent short circuits. The right thickness helps improve the mechanical strength and safety of the separator. However, if the separator is too thick, it increases the battery’s internal resistance, which affects charge-discharge performance.
Electrolyte
The electrolyte is made by mixing high-purity organic solvents, electrolyte lithium salts, and necessary additives in specific proportions. It plays a key role in conducting lithium ions. This is essential for the battery’s charging and discharging processes.
Common organic solvents include carbonates such as ethylene carbonate (EC), dimethyl carbonate (DMC), and methyl ethyl carbonate (EMC). These solvents effectively dissolve the electrolyte lithium salt. They provide an ideal environment for lithium ion migration. The key component of the electrolyte is the lithium salt. The most common lithium salt used is lithium hexafluorophosphate (LiPF₆). It offers high ionic conductivity and good electrochemical stability. A newer lithium salt, lithium bis(fluorosulfonyl)imide (LiFSi), has attracted attention. It has higher conductivity, better thermal stability, and resistance to hydrolysis.
Additives, though used in small amounts, play a crucial role. They can improve the battery’s charge-discharge efficiency. They also extend cycle life and enhance safety. For example, film-forming additives create a stable solid electrolyte interface (SEI) layer on the electrode surface. This prevents further reactions between the electrolyte and electrodes. It improves the battery’s cycle stability and safety. Flame-retardant additives reduce the flammability of the electrolyte. This enhances the battery’s safety performance.
Epic powder
In conclusion, Epic Powder is at the forefront of advancing lithium battery materials with a strong emphasis on ultra-fine grinding technology. Our specialized processes ensure the highest purity and particle size control for cathodes, anodes. By incorporating innovative grinding methods, we enhance the performance, efficiency, and safety of lithium batteries. Epic Powder is dedicated to delivering high-quality materials tailored to the needs of industries such as electric vehicles, energy storage, and consumer electronics, all while contributing to the evolution of energy storage solutions.

